Conduction
The transfer of thermal energy in solids or fluids at rest.1
Example:
When a hot glass or container is placed on a table and then removed some time later (depending on the conduction of the table) the table will be warm. Conduction occurs because of the temperature difference between the surfaces in contact.
Good conductors include metals such as aluminum, copper, iron, stainless steel, and nickel.
| Material | k (W/m K) |
| Aluminum | 121 - 237 |
| Copper | 61 - 401 |
| Iron | 51 - 80 |
| Stainless Steel | 13 - 64 |
| Nickel | 12 - 90 |
Insulators are materials that impede heat transfer (opposite of conductors).
Good insulators include fiberglass, polystyrene (Styrofoam), cork, and most ceramics.
|
Material |
k (W/m K) |
| Fiberglass | 0.036 - 0.046 |
| Polystyrene | 0.13 - 0.202 |
| Cork | 0.039 |
| Ceramics | 0.92 - 55 |
The governing equation for conduction is Fourier's Law:
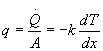
q = heat flux (W/m2)
![]() = heat transfer (W)
= heat transfer (W)
A = cross-sectional area (m2)
k = thermal conductivity (W/mĚK)
dT/dx = temperature gradient (K/m)