|
|
|
|
|
Next: 7.13 Reflection and Transmission Coefficients |
|
The motion of the wave packets in section 7.11 approximated that of classical Newtonian particles. However, if the potential starts varying nontrivially over distances short enough to be comparable to a quantum wave length, much more interesting behavior results, for which there is no classical equivalent. This section gives a couple of important examples.
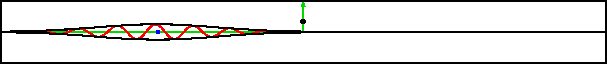
A classical particle entering a region of changing potential will keep going as long as its total energy exceeds the potential energy. Consider the potential shown in green in figure 7.19; it drops off to a lower level and then stays there. A classical particle would accelerate to a higher speed in the region of drop off and maintain that higher speed from there on.
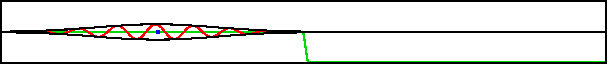
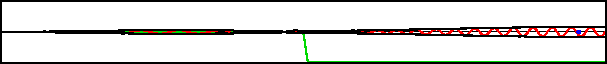
Improved resolution version (566 kB) |
However, the potential in this example varies so rapidly on quantum scales that the classical Newtonian picture is completely wrong. What actually happens is that the wave packet splits into two, as shown in the bottom figure. One part returns to where the packet came from, the other keeps on going.
One hypothetical example used in chapter 3.1 was that of sending a single particle both to Venus and to Mars. As this example shows, a scattering setup gives a very real way of sending a single particle in two different directions at the same time.
Partial reflections are the norm for potentials that vary nontrivially on quantum scales, but this example adds a second twist. Classically, a decelerating force is needed to turn a particle back, but here the force is everywhere accelerating only! As an actual physical example of this weird behavior, neutrons trying to enter nuclei experience attractive forces that come on so quickly that they may be repelled by them.
A classical particle will never be able to progress past a point at
which the potential energy exceeds its total energy. It will be
turned back. However, the quantum mechanical truth is, if the region
in which the potential energy exceeds the particle's energy is narrow
enough on a quantum scale, the particle can go right through it. This
effect is called tunneling.
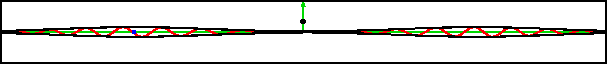
As an example, figure 7.20 shows part of the wave packet of a particle passing right through a region where the peak potential exceeds the particle’s expectation energy by a factor three.
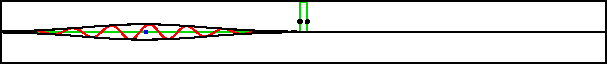
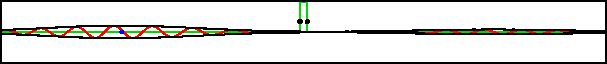
Improved resolution version (654 kB) |
Of course, the energy values have some uncertainty, but it is small. The reason the particle can pass through is not because it has a chance of having three times its nominal energy. It absolutely does not; the simulation set the probability of having more than twice the nominal energy to zero exactly. The particle has a chance of passing through because its motion is governed by the Schrödinger equation, instead of the equations of classical physics.
And if that is not convincing enough, consider the case of a delta
function barrier in figure 7.21; the limit of an infinitely
high, infinitely narrow barrier. Being infinitely high, classically
nothing can get past it. But since it is also infinitely
narrow, a quantum particle will hardly notice a weak-enough delta
function barrier. In figure 7.21, the strength of the delta
function was chosen just big enough to split the wave function into
equal reflected and transmitted parts. If you look for the particle
afterwards, you have a 50/50 chance of finding it at either side of
this impenetrable
barrier.
Improved resolution version (681 kB) |
Curiously enough, a delta function well, (with the potential going down instead of up), reflects the same amount as the barrier version.
Tunneling has consequences for the mathematics of bound energy states.
Classically, you can confine a particle by sticking it in between, say
two delta function potentials, or between two other potentials that
have a maximum potential energy ![]()
![]() .
.
Note however that in many cases, the probability of a particle tunneling out is so infinitesimally small that it can be ignored. For example, since the electron in a hydrogen atom has a binding energy of 13.6 eV, a 110 or 220 V ordinary household voltage should in principle be enough for the electron to tunnel out of a hydrogen atom. But don’t wait for it; it is likely to take much more than the total life time of the universe. You would have to achieve such a voltage drop within an atom-scale distance to get some action.
One major practical application of tunneling is the scanning tunneling microscope. Tunneling can also explain alpha decay of nuclei, and it is a critical part of much advanced electronics, including current leakage problems in VLSI devices.
Key Points
- If the potential varies nontrivially on quantum scales, wave packets do not move like classical particles.
- A wave packet may split into separate parts that move in different ways.
- A wave packet may be reflected by an accelerating force.
- A wave packet may tunnel through regions that a classical particle could not enter.